
Ask the AI Tutor
Need help with Periodic Table - Group 7 Elements? Ask our AI Tutor!
AI Tutor - Lucy
Connecting with Tutor...
Please wait while we establish connection
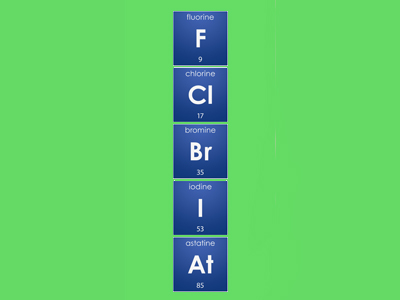
Reactivity of the elements in group 7 decreases down the group. This is because the electrons in the outer shell are further away from the nucleus.
Periodic Table - Group 7 Elements
Group 7 elements have seven outer electrons and form 1− ions. This GCSE Chemistry quiz covers trends, halogen reactions, and key tests for halide ions.
1 .
Group 7 elements only form what type of ion?
1+
7-
1-
2-
They all gain one electron to fill their outermost electron shell
2 .
Reactivity of the elements in group 7 decreases down the group. This is because...
There are more electrons in the outer shell
The electrons in the outer shell are further away from the nucleus
The electrons are closer to the nucleus
There are more electrons in the atom
The halogens react by attracting electrons from other atoms into the outer shell. As you descend the group, the atoms have more shells. These shield incoming electrons from the attractive force of the nucleus making it more difficult for the atoms to react
3 .
What type of bonds do group 7 elements form?
Ionic and covalent
Covalent only
Metallic and covalent
Hydrogen and metallic
They form ionic bonds with metals but can also form covalently bonded molecules with other non-metals
4 .
Choose the correct order from top to bottom of group 7.
F, Br, I, At, Cl
At, I, Br, Cl, F
F, Cl, Br, I, At
Br, Cl, I, At, F
It is useful to remember this order of elements as it the patterns of chemical and physical properties of group 7 usually refer to all or part of it
5 .
Halogens exist as molecules made up of pairs of atoms, joined together by covalent bonds. These molecules are known as...
Joined
Biatomic
Double
Diatomic
Di = two and atomic = atoms
6 .
How many electrons are in the outer shell of the group 7 elements?
7
8
1
2
At GCSE, the group number gives you the number of outer electrons
7 .
Iodine is...
a pale yellow gas
a dense green gas
a dark grey crystalline solid
a dark orange-brown solid
Iodine is poisonous and produces a violet coloured vapour when heated
8 .
Group 7 elements are also known as...
Alkali metals
Halogens
Transition elements
Noble gases
From the ancient Greek - halos (salt) and gen (producer)
9 .
Bromine displaces iodine from solution because...
Iodine is more reactive than bromine
Bromine is more reactive than iodine
Bromine is a smaller atom
Iodine has more electrons than bromine
Reactivity of the halogens decreases down the group
10 .
Halogens all react with metals. How many electrons do they gain when they undergo this type of reaction?
2
3
7
1
This gives them the stable electron configuration of the nearest noble gas
**Unlimited Quizzes Await You! 🚀**
Hey there, quiz champ! 🌟 You've already tackled today's free questions.
Ready for more?
Ready for more?
🔓 Unlock UNLIMITED Quizzes and challenge yourself every day. But that's
not all...
not all...
🔥 As a Subscriber you can join our thrilling "Daily Streak" against other
quizzers. Try to win a coveted spot on our Hall of Fame Page.
quizzers. Try to win a coveted spot on our Hall of Fame Page.
Don't miss out! Join us now and keep the fun rolling. 🎉
**Unlimited Quizzes Await You! 🚀**
Hey there, quiz champ! 🌟 You've already tackled today's free questions. Ready for more?
🔓 Unlock UNLIMITED Quizzes and challenge yourself every day. But that's not all...
🔥 As a Subscriber you can join our thrilling "Daily Streak" against other quizzers. Try to win a coveted spot on our Hall of Fame Page.
Don't miss out! Join us now and keep the fun rolling. 🎉






